Learning resources Discover more
What do you want to know more about? Choose your area of interest and find instructive material that will help you learn more about bladder and bowel care
What do you want to know more about? Choose your area of interest and find instructive material that will help you learn more about bladder and bowel care
key:global.content-type: Article
Ninthe (9) lives with her parents and brother in Almelo, The Netherlands. From the day she was born, she was unable to feel the urge to go to the bathroom. We interviewed Ninthe and her parents at their home. Ninthe hopes her story will inspire other children to understand that bowel irrigation is not a problem and that you can go to school again without having accidents.

Rosalinda, 67 years old and paralysed from the waist down, decided to swim the length of Loch Ness and became the first ever disabled person to do so. This was just one on a long line of swimming challenges that she has tackled – with great success, as her eight world records amply prove.
Veterans Affairs (VA) hospitals are going above & beyond by providing veterans with quality LoFric catheters for intermittent catheterisation (IC).

It can be difficult to find a regular toilet routine if you suffer from chronic constipation or fecal incontinence. Some people constantly fear public accidents or literary spending hours in the bathroom.

Although urethral catheter placement is routine after surgical procedures of benign prostatic hyperplasia (BPH), no guidelines inform the duration of catheter use. Results from ratings provided by a multidisciplinary panel in the US offer guidance in decreasing practice variation thereby reducing postoperative risks and improving the consistency and quality of care for patients undergoing surgery.
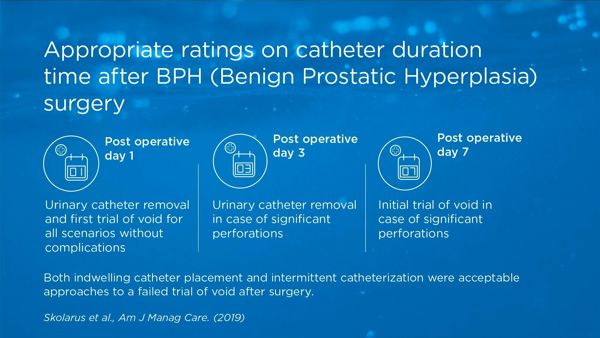
key:global.content-type: Article
Female urinary incontinence is a very common condition. It affects 1 in 4 women and means an involuntary loss of urine. As well as the physical effects of the condition, frequent accidents can lead to social discomfort or isolation. Let's take a look at what everyday life entails with respect to recognising and treating this issue, how can we recognise it and what solutions and treatments are available.

key:global.content-type: Article
In this blog, we will cover what urinary catheters are, why you would need to use them, and how they work. We’ll also look at some of the specific considerations for using catheters for both men and women.
A urinary tract infection (UTI) can affect any part of the urinary system and is caused by bacteria. Most commonly, the infection involves the lower urinary tract, which is the bladder and the urethra. Almost 90% of UTIs are caused by the bacteria 'Escherichia coli (E-Coli)' and this bacterium is often present in our gut without causing any harm.
key:global.content-type: Blogpost
Noah*, 49 years old, is recovering from rectal cancer. After surgery, he found he had additional problems with fecal incontinence which affected all aspects of his life. In this article, he tells us how Transanal Irrigation - TAI - allowed him to recover his freedom and confidence.

A burning sensation, sprints to the bathroom or just a little dribble? Symptoms from the lower urinary tract (LUTS) are extremely common in women, but nevertheless both unpleasant and embarrassing.

Marija, 58 – has been chronically constipated for years.

key:global.content-type: Article
Helene Skoog, June 14 2018 -- Many women with a Spinal Cord Injury, Spina Bifida, Multiple Sclerosis (MS) or injury from child birth have difficulties emptying the bladder. In this article we focus on the reason: Atonic Bladder.
